CUHK
News Centre
CUHK Uncovers How Helicobacter Pylori uses a Toxic Substance to Keep Alive in Human Stomach
A research team led by Prof. Kam-Bo Wong, Director and Professor of the School of Life Sciences at The Chinese University of Hong Kong (CUHK), has recently released its research results which show how a Helicobacter pylori (H. pylori) can survive in the human stomach by using a toxic metal, nickel ions, to activate an enzyme that can neutralize gastric acid. This discovery helps the future development of novel drugs against H. pylori infection. The team’s research findings were published today (5 Dec) in the Proceedings of the National Academy of Sciences of the USA, a prestigious international scientific journal.
H. pylori, which infects half of the human population and causes peptic ulcers and stomach cancer worldwide, is the only pathogen that can survive the gastric acidity in the human stomach. This is because H. pylori produces urease, a neutralising agent that breaks down urea into ammonia, which helps neutralise the acid. However, there is one problem for the bacterium which is that urease requires nickel ions to function. “Free nickel ions are toxic. H. pylori must find a way to deliver the nickel ions to the urease, without releasing the toxic metal ions inside the cells.” said Prof. Wong.
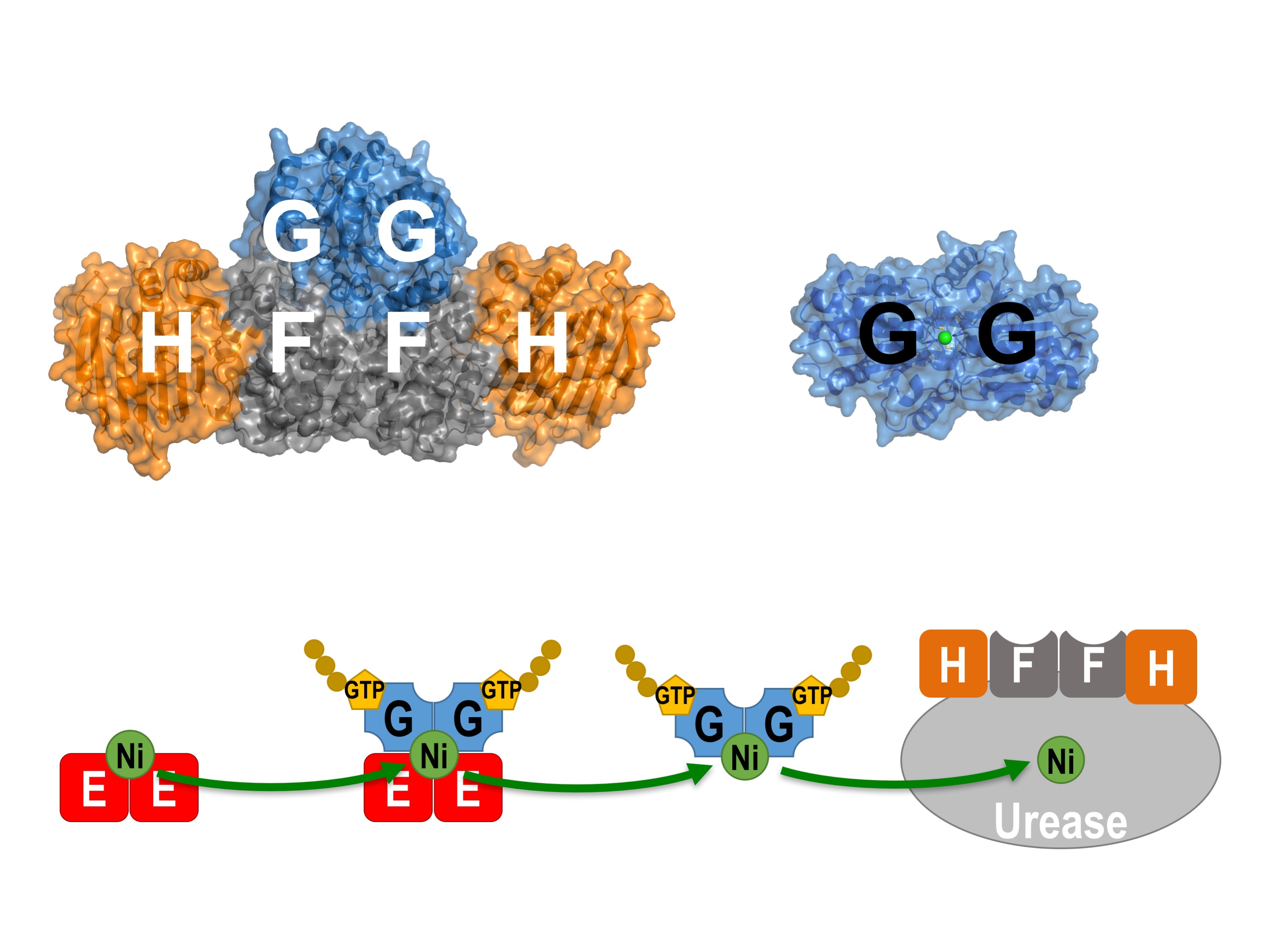
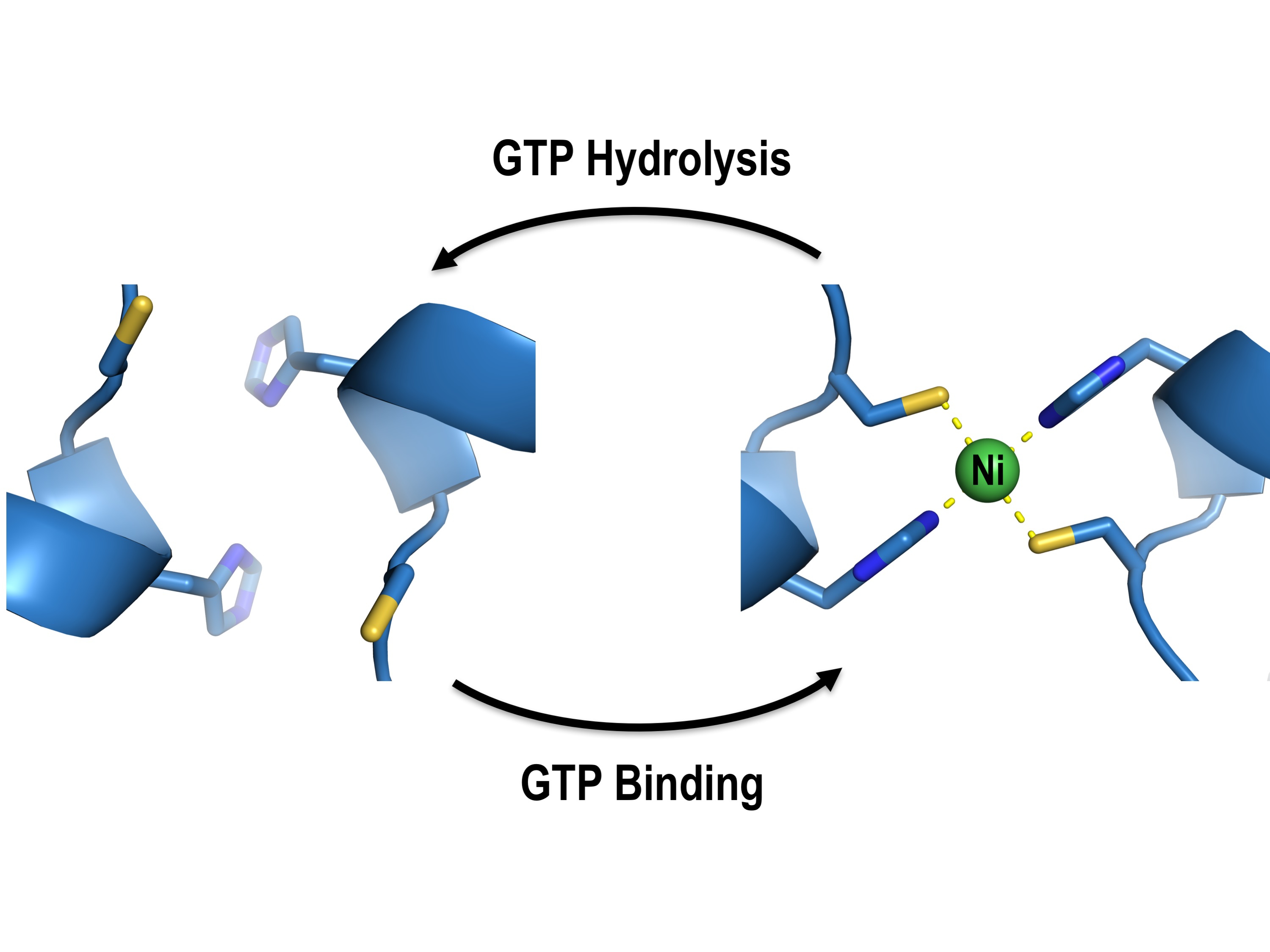
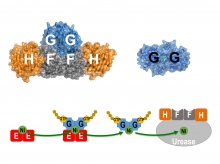
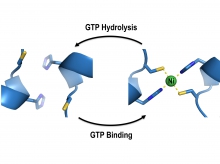
In H. pylori, the delivery of nickel ions for urease activation is assisted by four helper proteins, UreE, UreF, UreG and UreH. Prof. Wong and his team used X-ray crystallography as a molecular microscope to visualise how these helper proteins work together to deliver the nickel ions to the urease. They showed that the ability of UreG to change its molecular shape is essential for nickel delivery. Upon binding or hydrolysis of guanosine triphosphate (GTP), UreG can change its molecular shape, which determines its protein-interacting partners; UreG interacts with UreE when GTP is bound, but binds UreF/UreH after GTP hydrolysis. Prof. Wong said, “It allows the nickel ions to pass from UreE to UreG, and finally to the urease through protein-protein interactions so that the toxic nickel ions have no chance to escape inside the cells where they can create havoc.”
Since the survival of H. pylori depends on the production of active urease, Prof. Wong also sees the potential of inhibiting the nickel delivery pathway as a novel strategy to fight the H. pylori infection: “As H. pylori is increasingly resistant to antibiotics, we would like to find a new antibiotics target before the problem of resistance gets out of hand.” The team is now using structural information to screen drugs that inhibit urease activation.
Brief Biography of Prof. Kam-Bo Wong
Prof. Kam-Bo Wong is the Director of the School of Life Sciences at CUHK, and the deputy director of the Centre for Protein Science and Crystallography at CUHK. He is an expert in structure-function studies of proteins and has published in leading international journals such as Proceedings of the National Academy of Sciences of the USA, PLoS Biology, The Plant Cell, Nucleic Acids Research, and Journal of Biological Chemistry. He is also a member of the Partner State Key Laboratory of Agrobiotechnology at CUHK, and a team member of the two Areas of Excellence projects led by Prof. Liwen Jiang (Centre for Organelle Biogenesis and Function) and Prof. Hon-Ming Lam (Center for Genomic Studies on Plant-Environment Interaction for Sustainable Agriculture and Food Security) at the School of Life Sciences, CUHK.
Prof. Wong received his undergraduate training in biochemistry at CUHK, doctoral training at the University of Cambridge, and post-doctoral training at the University of Washington. His research work is supported by grants from the Hong Kong Research Grants Council and the Health and Medical Research Fund of the Food and Health Bureau of the HKSAR government. In addition to research, he is also devoted to education. He received the Science Faculty Exemplary Teaching Award in 2013, and is currently the Dean of Students at S.H. Ho College, CUHK.
Original Article: “Structural insights into how GTP-dependent conformational changes in a metallochaperone UreG facilitate urease maturation” (2017) Proc. Natl. Acad. Sci. USA http://www.pnas.org/cgi/doi/10.1073/pnas.1712658114
Prof. Kam Bo Wong and his research team. (From Left) Yap Shing Nim, Kam Bo Wong, Man Hon Yuen, and Yu Hang Fong.
Professor Wong and his team used X-ray crystallography as a molecular microscope to visualise how the helper proteins (UreE, UreF, UreG, and UreH) work together to deliver the toxic nickel ions to the urease, an enzyme essential for the infection of the H. pylori in the acidic human stomach.
After GTP hydrolysis, UreG changes its shape and releases its bound nickel ions, activating the urease with the help of UreF/UreH.