CUHK
News Centre
CUHK Engineering Develops New Technology Extending the Lifetime of Redox Flow Batteries and the Development of Grid-scale Energy Storage
A research team led by Professor Yi-Chun Lu, Associate Professor, Department of Mechanical and Automation Engineering, Faculty of Engineering at The Chinese University of Hong Kong (CUHK) has successfully developed a novel charge-reinforced ion-selective (CRIS) membrane for sulphur-based redox flow batteries, with 15 consecutive hours of runtime and over 2,000 hours cycling without obvious capacity decay. The new battery has taken a significant step forward in the practical application of redox flow batteries in grid-scale storage for renewable energy, and in its commercialisation, by resolving the problems posed by its poor lifetime and low cost-effectiveness. The breakthrough has been recently published in the world-leading scientific journal Nature Energy.
Conventional sulphur-based redox flow batteries with poor lifetime are not optimal for grid-scale energy storage
Aqueous redox flow batteries are a novel energy storage technology in which electricity is generated by electron transfer between two electrolytes. Compared to lithium-ion batteries, redox flow batteries are distinguished by the high safety, high power density and high flexibility in design which can be widely applied to devices including large-scale storage for renewable energy generated from solar or wind, and fast switching electric vehicles. However, the low energy density and high cost of conventional redox flow batteries determine their market penetration.
In 2016, a promising polysulfide-iodide redox flow battery was first invented by Professor Lu’s team taking advantage of the eco-friendly and low-cost sulphur element to greatly improve its energy density and cost effectiveness. However, the sulphur-based redox flow batteries adopting a commercial ion-selective membrane are compromised by the crossover and precipitation of active materials, leading to a rapid capacity decay and poor lifetime, and its application in large-scale energy storage fails to be effective. For example, a polysulfide-bromine project was launched by a UK technology company in the 1990s, but it was abandoned eventually due to its low energy density and poor lifetime. This led to a long-unsolved challenge for scientists.
CRIS membrane enables record stability and cycling
Based on the findings on the polysulfide-iodide redox flow batteries, Professor Lu has made efforts to improve the cycling stability and lifetime of the batteries. The team has designed the CRIS membrane, a simple and readily applicable incorporation of polymer-bounded carbon to create an ultra-thin cover, and place it on the commercial Nafion membranes (product model N117) to keep the two electrodes apart. The absorption of negatively charged species in porous carbon has strengthened the negative charge of the membrane and reduced the loss of active materials, which dramatically increased the stability and the lifetime of batteries. It is the first time in the world this goal has been achieved by using this new membrane, and it promises an effective application in grid-scale energy storage devices.
The demonstrated polysulfide-iodide redox flow batteries revealed an ultralow capacity decay rate (0.005% per day) for 1,200 cycles, and also achieved record high cycling stability and calendar lifetime with over 2,000 hours cycling (approximately 3 months), in comparison to only 160 hours cycling (approximately 6.7 days) with the commercial membrane N117, given that there is no obvious capacity decay. In addition, the coulombic efficiency of new batteries is more than 99.9% for every cycle with 15 consecutive hours of runtime on being fully charged, while there is 3 to 4 consecutive hours of runtime for sulphur-based redox flow batteries with a commercial membrane N117, and for lithium-ion batteries.
Under the condition in which the energy storage device with a new battery continuously operated and discharged for more than 15 hours, the surprising result yielded, in turn, a levelised cost of storage (LCOS) for this system which is competitive with other state-of-the-art redox flow techniques for long-duration energy storage applications. This approach can be universally applied in other redox flow batteries using sulphur-bromine or sulphur-iron materials, and in organic redox flow batteries, which goes further to fulfil a high stability of cycling.
Professor Lu said, “This approach successfully addresses the long-unresolved problems in the crossover of active materials in redox flow batteries and their poor lifetime. This encouraging membrane design strategy will enable practical commercialisation of sulphur-based redox flow batteries and guide the future development of highly-selective ion exchange membrane.”
About Professor Yi-Chun Lu
Professor Lu received her Bachelor degree in Materials Science and Engineering from the National Tsing Hua University, Taiwan, in 2007, and obtained a PhD in Materials Science and Engineering from the Massachusetts Institute of Technology (MIT), Cambridge, USA in 2012. She was in the first cohort of recipients of China’s Excellent Young Scientists Fund 2019 in recognition of her research work on electrochemical energy storage and material interface science. It is her goal to develop a stable, low-cost, scalable and rechargeable energy storage system.
Professor Lu is a Fellow of the Royal Society of Chemistry (RSC) and the Associate Editor of Journal of Materials Chemistry A published by the RSC. She is also a Founding Member of the Young Academy of Science of Hong Kong and had conferred on her various CUHK and international research and teaching awards. These include Top 10 Winners of Falling Walls Science Breakthroughs of the Year in Engineering and Technology (2020), United College Early Career Research Excellence Award, CUHK (2018), Young Researcher Award, CUHK (2016), the University Education Award, CUHK (2016), the Vice-Chancellor’s Exemplary Teaching Award, CUHK (2014), the Early Career Award, Research Grant Council (2014).
The full text of journal can be founded at: https://doi.org/10.1038/s41560-021-00804-x

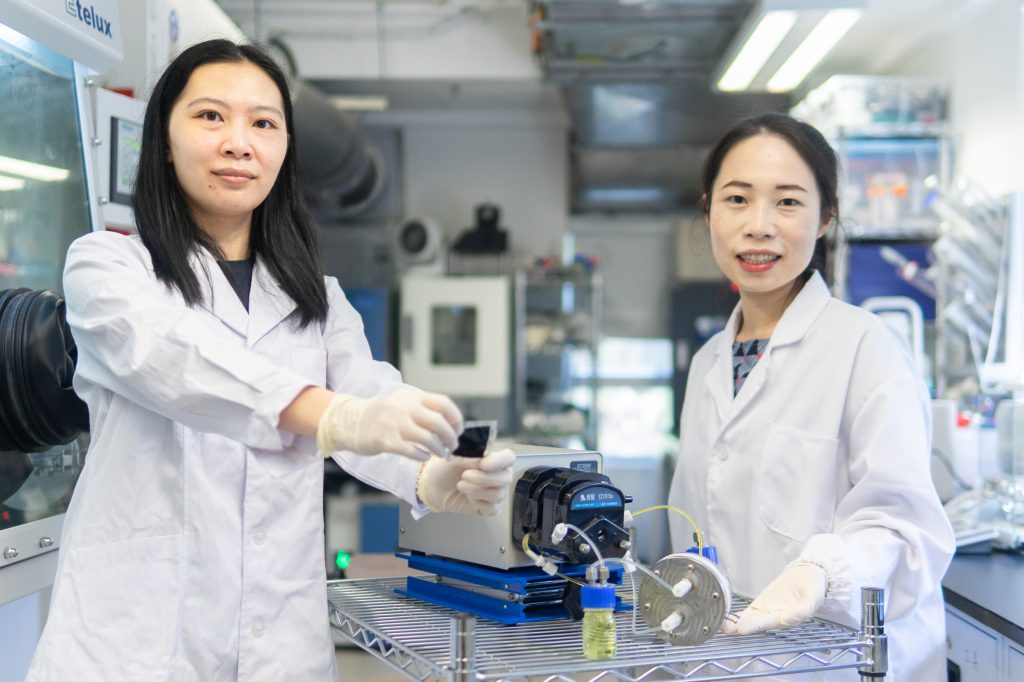
The CRIS membrane developed by Professor Yi-Chun Lu (right) and her team member Dr. Zhejun Li (left) can enhance the lifetime of sulphur-based redox flow batteries, and can be widely applied to large-scale storage devices.

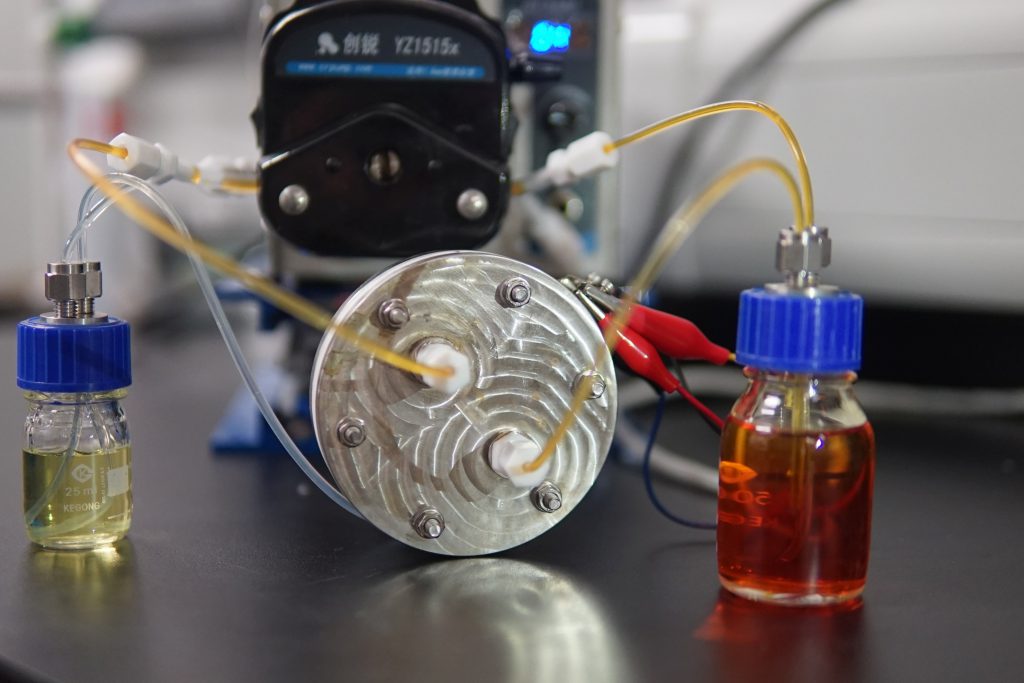
Crossover comparison between CUHK’s charge-reinforced ion-selective membrane (CRIS, right) and commercial Nafion membrane (N117, left).
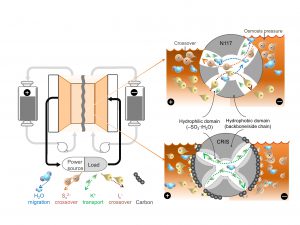
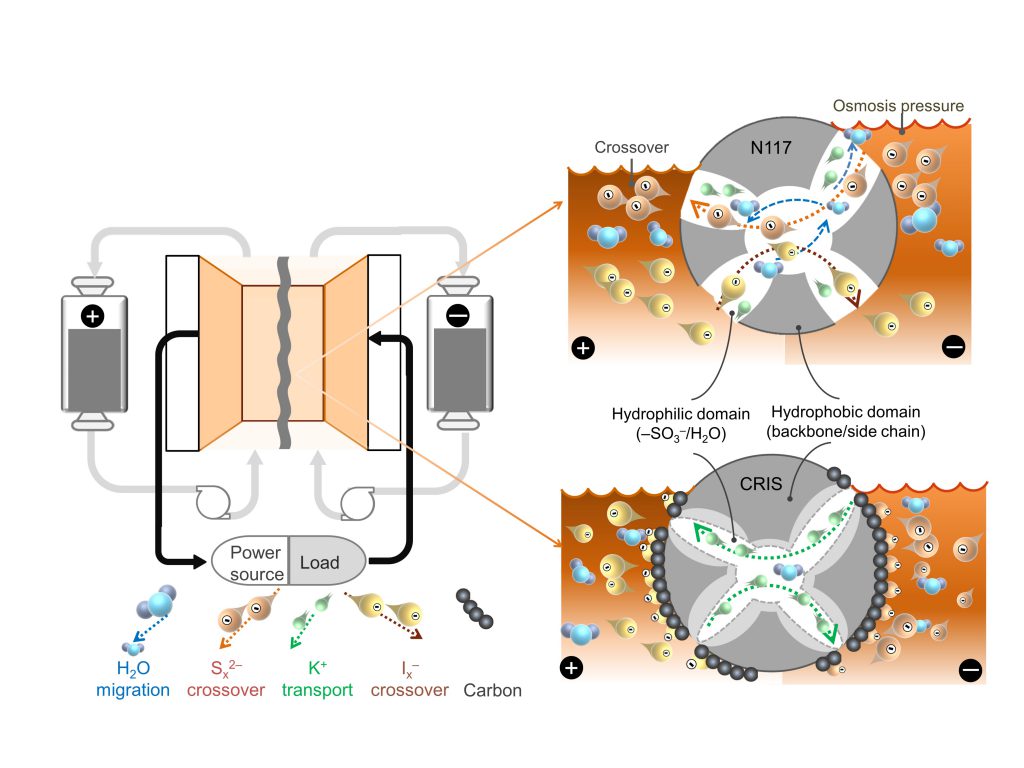
Diagram of a polysulfide-iodide redox flow battery. CRIS membrane repels the crossover of active materials themselves which dramatically improve the stability and lifetime of batteries, while the commercial membrane is challenged by the crossover of active materials which accelerates the capacity decay of battery.