CUHK
News Centre
CUHK unveils a new gene therapy targeting Parkinson’s disease
A team led by Professor Michael Kenneth Chan from the School of Life Sciences at The Chinese University of Hong Kong (CUHK) has recently developed a novel gene therapy to treat Parkinson’s disease (PD). The team found that a single injection of a recombinant adeno-associated virus (rAAV) expressing their patented therapeutic peptide in a targeted brain region could reduce neurodegeneration and ameliorate symptoms in preclinical PD animal models. The research findings have been published in Molecular Therapy, a leading journal in the field of gene and cell therapy.
A new approach to tackling Parkinson’s disease
Parkinson’s disease, the second most common neurodegenerative disorder, affects millions, with motor symptoms such as tremors, bradykinesia and rigidity, as well as non-motor manifestations such as sleep and mood disturbances. With no cure available, current treatments primarily alleviate symptoms, leaving an urgent unmet need for disease-modifying therapies that can slow or halt the neurodegenerative progression of the disease.
The pathogenesis of PD remains poorly understood but is believed to be associated with the aggregation of α-synuclein, an intrinsically disordered protein in the brain. Targeting this process has been challenging due to α-synuclein’s disordered structure and its complex aggregation pathway. Professor Chan’s team previously identified a small ubiquitin-like modifier (SUMO1)-derived peptide that specifically binds to monomeric α-synuclein, blocking the initiation of its aggregation cascade and exerting neuroprotective effects. As a protein originating from humans, SUMO1 also has low immunogenicity, enhancing its safety profile and potential as a therapeutic peptide inhibitor.
Transformative results in PD models
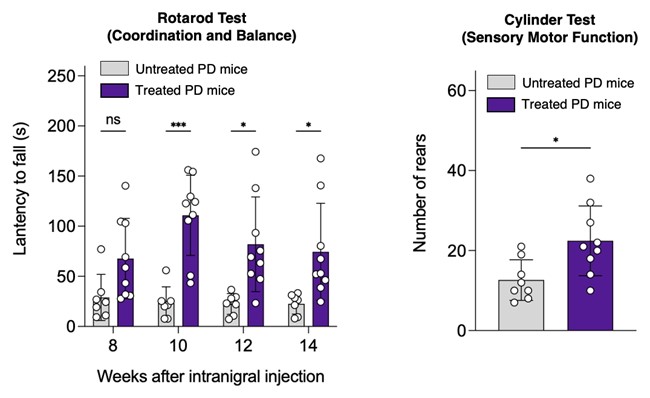
The team’s latest study validated the therapeutic efficacy of their modified SUMO1-derived peptide in both cell-based and transgenic fly PD models. They then used rAAV, a clinically established gene delivery vector, to achieve blood-brain barrier penetration and sustained expression of the peptide in a PD mouse model through a single intracranial injection. This allowed direct delivery of the modified peptide via rAAV to the targeted brain regions of a PD mouse. Experimental results showed that sustained expression of the peptide blocked α-synuclein aggregation, significantly alleviated PD-related motor deficits and protected dopaminergic neurons in the brains of a PD mouse model.
Professor Chan said: “This new gene therapy represents a breakthrough in Parkinson’s disease treatment. The demonstrated protection of dopaminergic neurons and reduced deterioration of motor function in PD mice following single injection of this therapeutic rAAV suggests that it could potentially be used to slow disease progression in PD patients. We hope to advance this technology toward clinical trials, and ultimately develop a treatment for PD that can help patients worldwide.”
With support from the Innovation and Technology Commission’s Technology Start-up Support Scheme for Universities, the team has established SUMO Therapeutics, a start-up focusing on advancing therapeutic strategies for effective treatment of PD and Lewy body dementia – two major neurodegenerative diseases in humans that are associated with α-synuclein aggregation. This entrepreneurial step underscores their commitment to translating cutting-edge research into solutions to global health challenges.