CUHK
News Centre
CUHK Uncovers Molecular Machine that Keeps Helicobacter pylori Alive in Human StomachOpens up Novel Strategy to Eradicate the Culprit of Stomach Ulcers and Cancer
A research team led by Prof. Kam-bo Wong of the Centre for Protein Science and Crystallography, School of Life Sciences at The Chinese University of Hong Kong (CUHK), has released its research results which show how a bacterium, Helicobacter pylori, that infects half of the human population, causing peptic ulcers and stomach cancer worldwide, has managed to survive in the acidic environment of the human gut. The research paper has been selected as ‘Paper of the Week’ in Journal of Biological Chemistry, for its important implications in the design of new drugs to blunt H. pylori’s impact on human body.
H. pylori is the only bacterium known to thrive in the human stomach. It damages the mucous coating of the gut, allowing stomach acid to eat away the sensitive organ lining and causing ulcers. Existing antibiotics can cure 80 to 90 percent of ulcers caused by pathogens. However, H. pylori has become increasingly resistant to antibiotics over the years.
‘There is a pressing need to develop new drugs and alternative strategies to fight against H. pylori infection bef ore the prevalence of antibiotic resistance gets out of hand,’ said Ivan Fong, a member of Professor Wong’s research team. ‘The key to the survival of H. pylori in the acidic bath in the human stomach is its use of an enzyme called urease to neutralize gastric acid.’
H. pyloriproduces urease to spur the breakdown of urea, a naturally occurring chemical in the body, so that urea can release ammonia to neutralize the acidic environment in the gut, allowing pathogens to thrive. Unlike most other enzymes, urease does not work immediately after being produced by the bacterium. Two nickel ions have to be delivered to activate it.
‘As the survival of H. pylori depends on active urease, this is a life-or-death issue for the pathogen to ensure nickel ions are delivered to the urease,’ says Professor Wong, ‘so our team focused on studying the four proteins that help the activation of urease: UreE, UreF, UreG and UreH.’
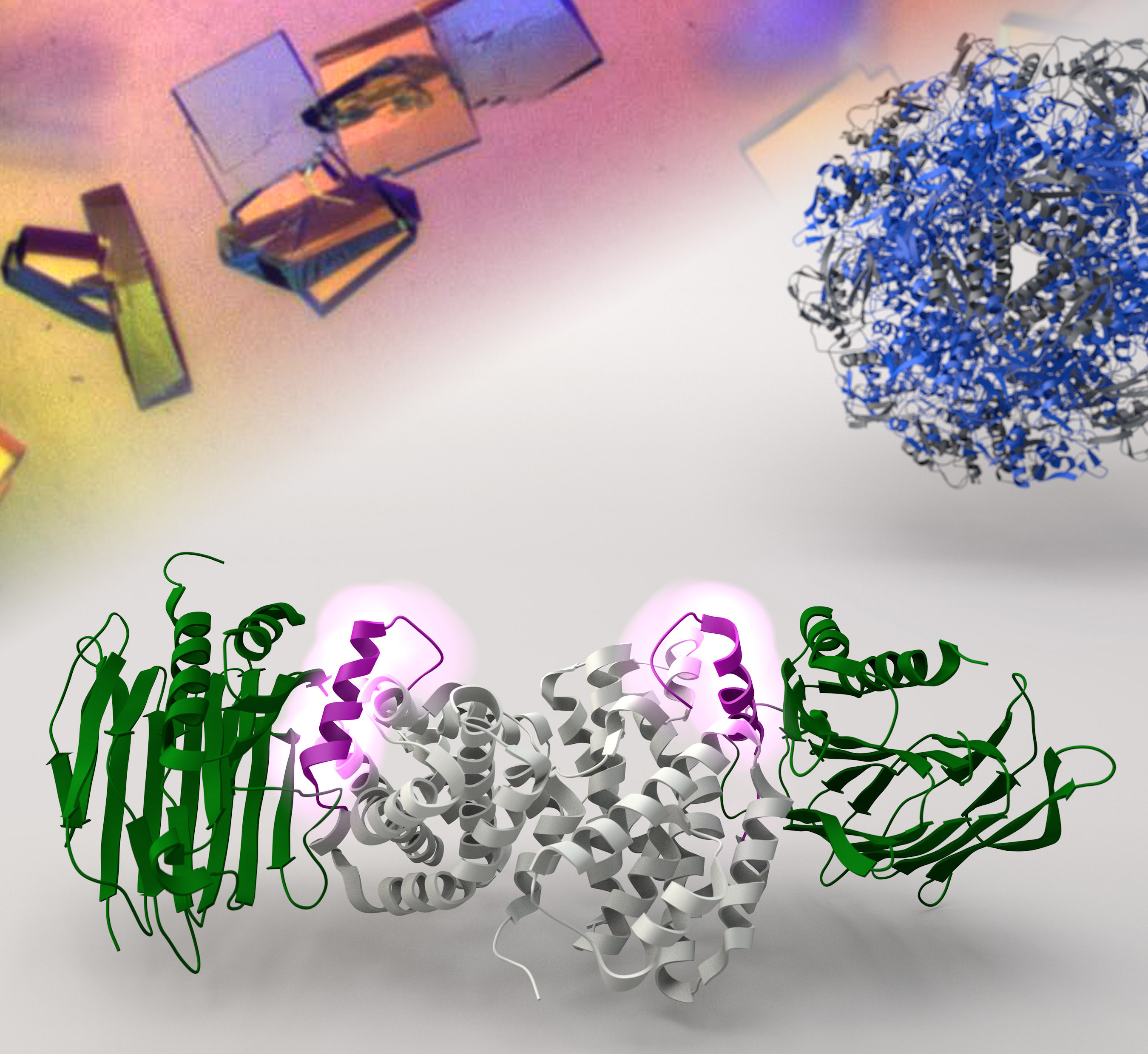
Professor Wong and his team used X-ray crystallography, which essentially functions as a molecular microscope to visualize proteins with atomic resolution, to observe how the helper proteins UreF, UreH and UreG hook up collectively to assemble a molecular machine that delivers nickel ions to urease. Once the nickel ions are in place, the breakdown of urea into ammonia will start immediately to neutralize the stomach acids.
More importantly, the team also demonstrated that disrupting the formation of the UreF-UreH-UreG complex can inhibit the synthesis of active urease. Professor Wong said, ‘With a better understanding on how the molecular machine is assembled, we can now proceed to study ways to dissemble it. As active urease is the key to the survival of H. pylori, new drugs designed to target this complex may well be a novel and viable strategy to eradicate the pathogen.’ The team is now working on the design of drugs that inhibit assembly of this molecular machine that keeps H. pylori alive in human stomach.