CUHK
News Centre
CUHK Chemists Devise a Novel Catalytic System to Help Design Chemical Compounds Enriches Chemistry Toolkit for Advanced Drug Synthesis
Drug synthesis is the process of designing and combining chemical components that can be applied to target specific illnesses. The designing is often similar to building a model. If the model requires a specific piece that cannot be made, the design becomes impossible. Similarly, new, never-been-made chemical motifs are sometimes needed in drug designs.
Recently, researchers from the Department of Chemistry at The Chinese University of Hong Kong (CUHK) have been the first to devise a novel halocyclization-based catalytic system for the preparation of chiral halo-spiro compounds, a type of chemical with great potential in drug discovery. This enriches the chemistry toolkit used by the pharmaceutical industry for new drug design options. The research finding has been published in the renowned science journal Nature Catalysis.
Nature often has a preference for either left or right
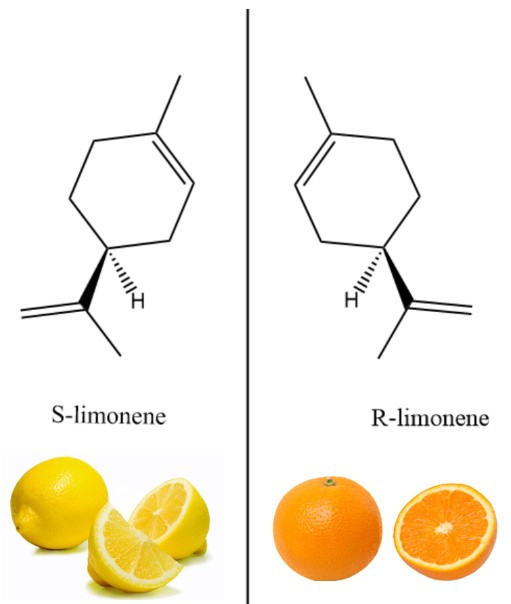
By mere translations and rotations, our left hand cannot look the same as our right hand. Analogously, many compounds look very similar but are different in the left/right symmetry, and cannot superimpose its mirror image by rotations or translations. Compounds with these characteristics are said to be chiral. The chemical differences between the left and right compounds sometimes have different physiological properties. For instance, a “left” compound S-limonene smells like lemons, whereas its “right” counterpart R-limonene smells like oranges (see image 1).
In medicine, different chiral compounds can mean life or death. Therefore, being able to control the chirality of drug molecules is critical, but it has been a long-standing challenge because the structural similarity of the chiral compounds often causes tremendous difficulty in creating and separating specific chiral compounds. Most current approaches in producing chiral compounds target structurally simple molecules, leading to limited applications.
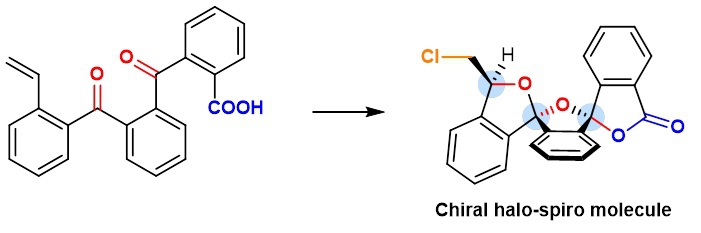
By combining computation and experiment, a team led by Professors Ying-Lung Steve TSE and Ying-Yeung YEUNG from the Chemistry Department at CUHK, has devised a novel catalytic system for the preparation of chiral halo-spiro molecules (see image 2). These molecules have intrinsic complexity and, more importantly, rigidity scaffolds which offer several advantages to drug discovery work. The addition of halogen atoms to the spiro compounds permits a significantly greater degree of freedom in drug design.
The heart of the catalytic system is the theoretical calculations carried out by Professor Tse. The calculations were based on quantum mechanics. Professor Tse said, “These calculations have to be very accurate, but they also tend to be very computationally expensive. We have improved the efficiency by making clever approximations without sacrificing accuracy. By performing more efficient, cleverer calculations, we will be able to incorporate even more realistic conditions and better compare our numbers with the experiments. The future is certainly going to be very exciting for both experiment and computation in chemistry and drug discovery.”
About Professor Ying-lung Steve TSE
Professor TSE and his research group have been using theory and computation to study different chemical problems. In collaboration with Professor YEUNG, the team is interested in explaining how the chemical species, called catalysts, are able to speed up organic reactions that are intrinsically too slow to happen without catalysts.
Professor TSE obtained his Ph.D. degree at Stanford University and postdoctoral at the University of Chicago.
A full version of the paper can be found at:
https://www.nature.com/articles/s41929-020-00530-9
Image 1: Many compounds are naturally chiral and different in the left/right symmetry. For instance, a “left” compound S-limonene smells like lemons, whereas its “right” counterpart R-limonene smells like oranges.
Image 2: The team led by Professors Ying-Lung Steve TSE and Yeung-Ying YEUNG successfully synthesized chiral halo-spiro molecules and explained how the process works.